The atomic and electronic structure of advanced oxide materials, with emphasis on calculations of properties of defects and surfaces
Complex oxides e.g. ABO3-type perovskites have enormous range of applications, including spintronics, catalysis, electrochemical applications, e.g., cathodes of solid oxide fuel cells (SOFC), ceramic membranes for gas separation, actuators, sensors, etc. Mixed oxides with the ABO3 perovskite structure are flexible systems as their properties can be adjusted or enhanced for specific applications by chemical doping at the A or B cation sites. These nonstoichiometric oxides contain point defects in the form of vacancies and trapped electrons/ holes depending on the A, B cation- and dopant nature. In our first principles calculations we focus on defects and surface properties which are important for high tech applications.
Using the hybrid DFT-HF approach as implemented into the CRYSTAL code, we performed large-scale supercell computer calculations of bulk and surface O vacancies with trapped electrons (known as the F centers) in three key perovskite crystals: SrTiO3, PbTiO3 and PbZrO3.[1,2]. The local lattice relaxation, charge redistribution and positions of defect levels within the band gap have been compared. We have demonstrated that difference in a chemical composition of host materials leads to quite different defect properties: the F center is a shallow defect in titanates but a deep defect in zirconite. All three perovskites show a considerable trend in O vacancy segregation to the surfaces which important for the interpretation of the experimental data on mass-charge transport in nanocrystalline materials. The same trend of defect segregation towards surfaces (or internal grain boundaries) was observed in our joint study with Max Planck Institute FKF in Germany for the charged F+ centers in SrTiO3 (O vacancy with a single trapped electron).
Based on modeling defect energetics on LaMnO3 doped with Sr (LSM), we developed possible scenarios of the oxygen reduction on the SOFC cathode [3] which opens the way for material and device further optimization.
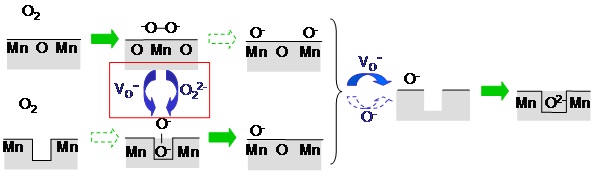
Figure : The most probable mechanism of oxygen incorporation on MnO2[001]-terminated La1-xSrxMnO3 follows the solid arrows. Straight arrows indicate reaction steps (chemisorption, dissociation, Oincorporation), bent arrows describe transport (diffusion parallel to the surface). The rate-determining step in the red box is the encounter of an adsorbed molecular oxygen species O2- or O22- and a surface oxygen vacancy.
Currently, we are working on more complex multi-component materials such as (Ba,Sr)(Co,Fe)O3 in order to optimize their composition and properties.
References:
1. Yu. Zhukovskii, E.A. Kotomin, R.A. Evarestov, D.E. Ellis, Int. J.Quant.Chem. 107, 2959-2985 (2007)2. Yu. Zhukovskii, E.A. Kotomin, D.E. Ellis, Solid state comm.., 149, 1359-1362 (2009).
3. Yu. Mastrikov, R.Merkle, E.Heifets, E.A.Kotomin, J.Maier, J.Phys.Chem. C 114, 3017-3027 (2010).