Thermodynamics of advanced materials
The first-principles calculations are performed at zero K and additional effort is necessary to compare these results with the experimental data obtained under realistic conditions, e.g. high temperatures (ca. 800 C for fuel cells) and high partial gas pressure. To fill this gap, we developed ab initio thermodynamics of advanced oxide materials. Based on this approach, we are able to predict range of stability for different oxide terminations (pure and with adsorbates) which is a key issue for many applications. In particular, we have performed detailed calculations of the atomic, electronic structure and thermodynamic stability of La1-xSrxMnO3 (LSM) solid solutions and its parent compound LaMnO3 (LMO) and now study more complex (Ba,Sr)(Co,Fe)O3 solid solutions.
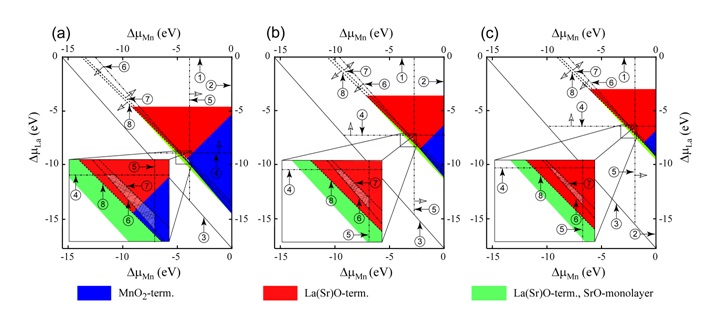
Fig. Sections of thermodynamic stability diagram for LSM (001) surface structures for O2 partial pressure (p = 0.2p0) and temperatures of (a) 300 K (room temperature), (b) 1100 K (SOFC operational temperature), and (c) 1500 K (sintering temperature). The region, where LSM (x = 1/8) is stable, is the hatched area between LaMnO3, La2O3, Mn2O3, and SrO precipitation lines. The numbers from 1 to 8 in the circles indicate precipitation lines for (1) La, (2) Mn, (3) Sr, (4) La2O3, (5) Mn2O3, (6) SrO, (7) LaMnO3, and (8) SrMnO3. Hollow arrows indicate the sides from respective precipitation lines where the precipitation occurs. Insets show magnified areas with the region of LSM stability (a hatched quadrangle).
References:
1. S. Piskunov, E.Heifets, T.Jacob, E.A.Kotomin, D.E.Ellis, and E.Spohr, Phys. Rev. B 78, 121406 (2008).
2. Yu. Mastrikov, E. Heifets, E.A. Kotomin, J. Maier, Surf. Sci. 603, 326 (2009).
3. E.A. Kotomin, Yu. Mastrikov, E. Heifets, J. Maier, Phys Chem Chem Phys. 10, 4644 (2008).
