Development of Methods for a study Fluctuation-Controlled Kinetics
From a formal point of view, spatially homogeneous condensed system with chemical reactions is similar to other homogeneous many-particle systems like gases and liquids. All these systems may be described by the same sets of structural characteristics. It is hereby not essential that in chemical systems the number of particles (reactants) may change and that non-equilibrium processes occur. Such factors determine only the specific form of theoretical equations, the basic structural characteristics are conserved. Many-particle systems may be characterized, in general, by a developed spectrum of reactant density fluctuations. The correct description of such spectra may require the determination of an infinite number of different functions. It is therefore reasonable to start with the most simple structural characteristics. These are the macroscopic concentrations, i.e., the densities of particles ni(t) The quantity ni(t)is a measure of the average distance between the particles. As a next step in the description, pair correlation functions Fij(r,t) may be introduced, known in the statistical physics of dense gases and liquids as radial distribution functions. The knowledge of the pair correlation functions allows the determination of the dispersion of the number of particles in an arbitrary volume.
For many--particle systems of any kind it is found that the equations determining the structural characteristics consist of a infinite chain of coupled BBGKY-like equations. This is valid also for chemically reacting systems [1-3]. The accurate equation for the macroscopic concentration contains in the right hand side (via the reaction rate constants) the pair correlation functions. The pair correlation functions are determined via triple correlations etc. By this reason, the set of equations can be solved only using certain approximations. For the physics of many-particle systems, a reduced description of the spectrum of fluctuations is usually applied by cutting the infinite set of equations. This is also the case in the description of the kinetics of bimolecular reactions. The reduced description is introduced by applying only the simplest structural characteristics like the macroscopic concentrations and the pair correlation functions [1-3]. Hereby Kirkwood's approximation is used, where the triple correlations are expressed via the pair correlation functions. The comparison of two approaches (microscopic theory and computer simulation) shows that the reduced description of the density fluctuation spectrum of the system does not lead to considerable mistakes in the determination of the correlation functions.
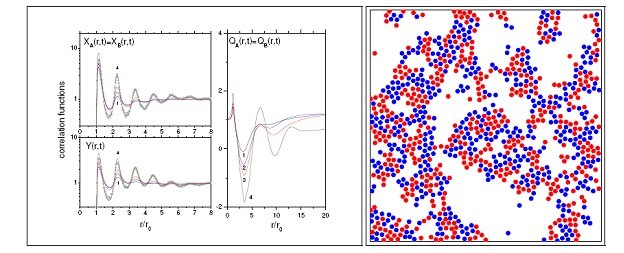
Fig.1. A system with a strong Coulomb interaction and short-range (Lennard-Jones) potential. Correlation functions plotted on the left panel demonstrate a series of peaks corresponding to the formation of the first, second, third etc coordination spheres typical for condensed matter. The middle window shows the screening parameter. The right window shows the typical particle distribution restored using Reverse Monte Carlo method.
References:
- H. Schnorer, V.N. Kuzovkov, and A. Blumen, Segregation in Annihilation Reactions without Diffusion: Analysis of Correlations. - Physical Review Letter, 1989, 63, p.805-808
- V.N. Kuzovkov and E.A. Kotomin, Kinetics of Bimolecular Reactions in Condensed Media. - Rept. Progr. Phys., 1988, 51, p. 1479-1524.
- E.A. Kotomin and V.N. Kuzovkov, Phenomenological Theory of the Recombination and Accumulation Kinetics of Radiation Defects in Ionic Solids. - Rept. Progr. Phys., 1992, 55, p. 2079-2202.